Glide, Part I: Azeotropic and zeotropic mixtures
Refrigerant fluids can be one component pure fluid, such as R134a and R1234ze, or mixtures of two or more refrigerants, such as R507, R410A, R407C and R455A.
By mixing appropriately selected fluids and introduced into the formulation in a suitable concentration, the researchers try to mitigate the penalizing characteristics of one of the refrigerants present in the mixture, such as, for example, flammability, toxicity, global warming potential, inadequate miscibility with the compressor lubricant, and at the same time preserve, within suitable limits of use, the characteristics that make these molecules suitable for use as working fluids in steam compression machines.
During the phase change, the mixtures may or may not exhibit the typical behaviour of pure fluids: the former are defined as azeotropic (R507, among those mentioned above), zeotropic, or non-azeotropic, the latter (from the previous list, R410A, R407C and R455A).
More in detail, azeotropic mixtures are the ones that at constant pressure change phase at a constant temperature: within a refrigerant circuit their behavior will therefore be identical to that of pure fluids, ignoring the effects resulting from the possibility of variation in composition for different solubility of the components in the lubricating oil.
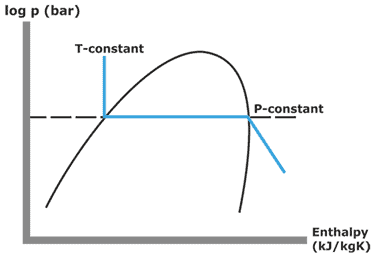
Limiting the analysis to two-component mixtures for ease of handling, the liquid-vapour equilibrium diagram for azeotropic mixtures takes the form of the type illustrated below:
The upper line, which delimits the vapour zone, is called dew curve (or, better, due point) and is drawn by diagramming, as the composition of the mixture changes, the temperature value at which the first trace of liquid appears when the mixture at the vapour state is cooled by an isobaric process. The lower curve is called the bubble curve, and with a similar method, it is drawn by collecting the temperature values in which the first bubble of vapour appears in the isobaric heating of the mixture in liquid phase.
When it happens that, as represented in the diagram, for a certain value [better to say “for a limited range of values”] of composition the bubble and dew curves join, we have, at that concentration value, an azeotropic mixture.
At this composition, the isobaric change of phase takes place, as clearly deduced from the diagram, also isothermally.
The azeotropic composition is actually subject to variations as the pressure changes. However, within the usual pressure ranges within which the refrigeration cycles operate, a mixture of a suitable intermediate composition can be considered, with a good approximation, azeotropic both at the condenser and at the evaporator.
Written by Francesco Viola, Eng.