Glide, Part III: Practical application
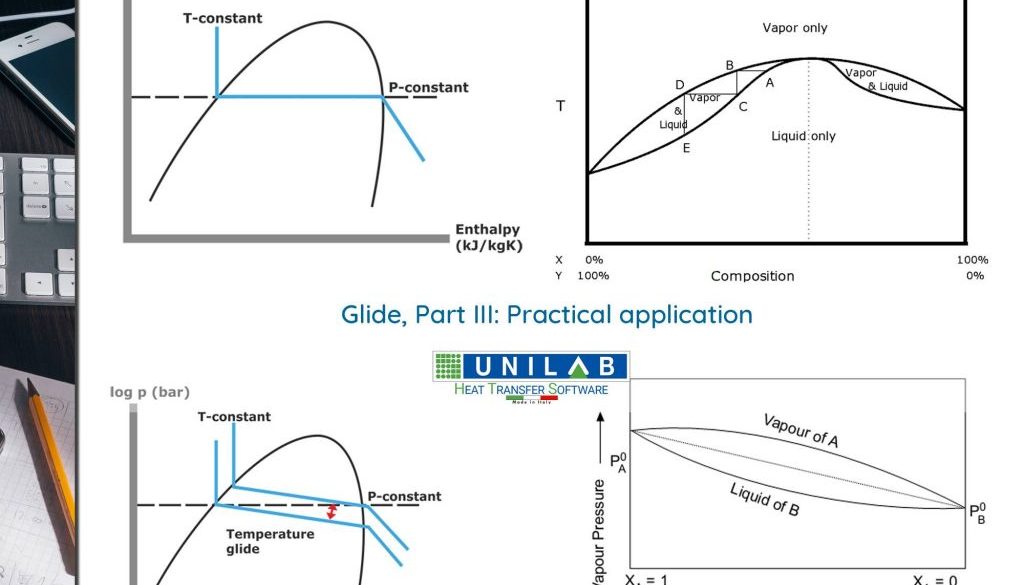
In the previous article of this series we had indicated that, as long as a zeotropic mixture has glide values lower than 2°K, its use in refrigeration circuits does not pose significant problems compared to the use of “homologous” one-component refrigerating fluids or in an azeotropic mixture (as in the case of R410A, for example), to the point that we sometimes speak of quasi-azeotropic mixtures. On the other hand, when the temperature glide is higher than the mentioned value, it is no longer allowed to ignore this circumstance in the design of the refrigerating circuit and in the operations connected to it.
For example, it is necessary to reconsider the configuration of the evaporator and the condenser in order not to suffer penalisations induced by the variation of the temperature of the fluid during the phase change. It is also necessary, on the other hand, to take advantage of this circumstance, creating circuit configurations that better approximate the counterflow regime.
In fact, the fact that the change of phase does not occur isothermically can be positively evaluated, as in this way it is generally possible to better tune the temperature profiles of the two fluids to the condenser and to the evaporator, making the temperature difference between the two fluids more uniform along the exchangers. In fact, this represents an advantage, since with the same average temperature of phase change and with the same overall heat transfer coefficient, it is possible to increase the thermal flow as a consequence of a higher value of the average logarithmic temperature difference between the two fluids.
However, we must not overlook the penalization of the heat transfer coefficient that results from the use of a zeotropic mixture with respect to pure fluids, of an entity approximately greater the higher the temperature glide in the phase change. Zeotropic mixtures are generally disadvantaged compared to pure fluids, for the purposes of heat transfer in phase change, due to the concentration gradients established near the exchange surfaces and that introduce diffusive heat resistances.
To better understand this phenomenon we can consider, as an example what happens to the condenser. In the condenser, the high boiling fraction of the mixture separates first, and near the exchange surface, the vapour phase mixture is enriched in the fractions which are more difficult to condense and which a similar “effect” when air infiltrations appear inside the condensers for pure fluids. The experimentation has shown that in these cases, high velocities of vapour crossing inside the tubes (values not less than 7 m/s) are effective in mitigating the effects of diffusive resistance due to the effect of turbulence triggered on the tubes’ walls (mixing).
Francesco Viola, Eng.